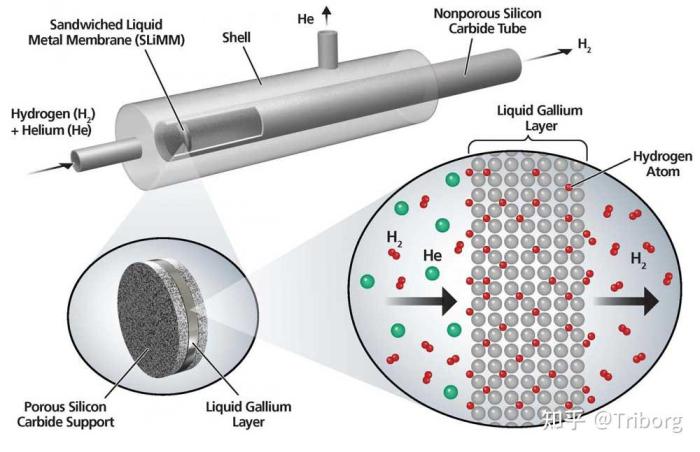
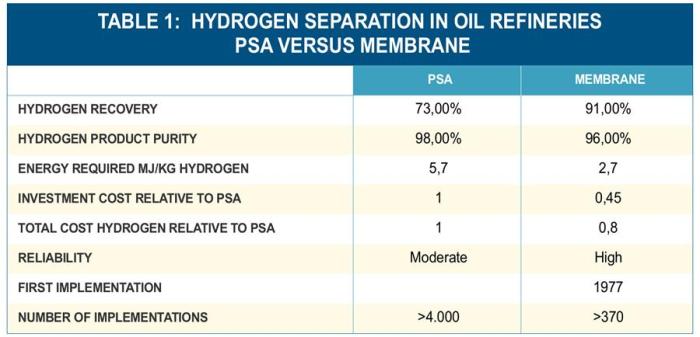
Osmoses hydrogen membrane separation – Osmosis hydrogen membrane separation, a cutting-edge technology, harnesses the power of selective membranes to purify and separate hydrogen gas from various mixtures. This process, driven by the principle of osmosis, allows hydrogen molecules to pass through a specialized membrane while effectively blocking other gases. Imagine a futuristic world where clean hydrogen fuels our vehicles and industries, a world powered by this innovative technology.
The heart of this technology lies in the membrane itself, a carefully engineered barrier that acts as a molecular sieve. Different types of membranes, from palladium-based to polymer and mixed matrix, each possess unique properties that influence their efficiency and suitability for specific applications. These membranes are the key players in the hydrogen revolution, paving the way for a cleaner and more sustainable energy future.
Introduction to Osmosis Hydrogen Membrane Separation: Osmoses Hydrogen Membrane Separation
Osmosis hydrogen membrane separation is a promising technology for producing high-purity hydrogen. This method leverages the selective permeability of a membrane to separate hydrogen from a gas mixture, utilizing the principle of osmosis.
The process relies on the difference in chemical potential between the feed gas and the permeate side, driving the selective transport of hydrogen through the membrane. This driving force can be created by applying a pressure difference across the membrane or by utilizing a concentration gradient.
Membrane Materials
The heart of this technology is the hydrogen-selective membrane. These membranes are typically thin films composed of specific materials that exhibit high permeability to hydrogen while effectively blocking other gases. Common materials include:
- Palladium alloys: These alloys, known for their excellent hydrogen permeability, have been extensively studied and utilized in hydrogen separation. However, their high cost and susceptibility to poisoning by impurities limit their widespread adoption.
- Metal-organic frameworks (MOFs): MOFs offer a highly porous structure with tunable properties, allowing for selective hydrogen permeation. Their potential for high hydrogen flux and stability makes them attractive candidates for membrane applications.
- Polymer membranes: Certain polymers, like polyimides and polysulfones, exhibit good hydrogen permeability and can be fabricated into thin, robust membranes. These materials are often blended with other polymers to enhance their performance and stability.
Driving Force for Hydrogen Separation
The driving force for hydrogen transport across the membrane is crucial for efficient separation. Two primary mechanisms are employed:
- Pressure difference: Applying a pressure difference across the membrane drives hydrogen from the high-pressure feed side to the low-pressure permeate side. This method is commonly used in industrial applications.
- Concentration gradient: Utilizing a concentration gradient, where the hydrogen concentration is higher on the feed side than on the permeate side, drives hydrogen transport through the membrane. This method is particularly useful for separating hydrogen from dilute gas mixtures.
History and Applications
The development of hydrogen membrane separation technology dates back to the early 20th century with the use of palladium membranes. However, the high cost and limited scalability of these materials hindered their widespread adoption.
Recent advancements in membrane materials, particularly in MOFs and polymer membranes, have revitalized interest in this technology. Applications of osmosis hydrogen membrane separation are expanding across various industries, including:
- Hydrogen production: Separating hydrogen from synthesis gas (a mixture of hydrogen, carbon monoxide, and other gases) produced by steam methane reforming or gasification.
- Hydrogen purification: Removing impurities like methane, nitrogen, and carbon dioxide from hydrogen streams to achieve high purity for fuel cells or other applications.
- Biogas upgrading: Extracting hydrogen from biogas, a renewable source of energy, for use as a fuel or feedstock.
Membrane Materials for Hydrogen Separation
The heart of hydrogen membrane separation lies in the membrane itself. These selective barriers allow hydrogen to pass through while blocking other gases, leading to a purified hydrogen stream. The choice of membrane material is crucial, as it directly impacts the performance and economics of the separation process. Let’s delve into the different types of membranes used for hydrogen separation and their unique characteristics.
Palladium-Based Membranes
Palladium-based membranes have long been recognized as the gold standard for hydrogen separation. Their exceptional hydrogen permeability and selectivity stem from the unique ability of palladium to absorb and transport hydrogen atoms through its lattice structure. This phenomenon, known as “palladium hydride formation,” results in a highly efficient hydrogen transport mechanism.
Properties and Characteristics
- High Permeability: Palladium-based membranes exhibit remarkably high permeability to hydrogen, significantly exceeding that of other membrane materials. This allows for efficient hydrogen transport and high separation rates.
- Excellent Selectivity: Palladium’s affinity for hydrogen is significantly higher than its affinity for other gases, resulting in excellent selectivity for hydrogen separation. This ensures a high purity of the separated hydrogen stream.
- High Stability: Palladium-based membranes are known for their stability at elevated temperatures and pressures, making them suitable for demanding industrial applications.
Advantages and Disadvantages
- Advantages:
- High permeability and selectivity for hydrogen.
- Excellent stability at high temperatures and pressures.
- Mature technology with established applications.
- Disadvantages:
- High cost due to the scarcity and processing of palladium.
- Susceptibility to poisoning by impurities such as carbon monoxide and sulfur compounds, which can hinder hydrogen transport.
- Brittleness and difficulty in forming thin, defect-free membranes.
Polymer Membranes
Polymer membranes offer an alternative to palladium-based membranes, providing a cost-effective solution for hydrogen separation. These membranes are typically made from organic polymers with specific properties that allow selective hydrogen transport.
Properties and Characteristics
- Moderate Permeability: Polymer membranes generally exhibit lower permeability to hydrogen compared to palladium-based membranes. However, they offer a balance between permeability and selectivity.
- Good Selectivity: Polymer membranes can achieve good selectivity for hydrogen, effectively separating it from other gases in the feed stream. This is achieved through careful selection of the polymer material and membrane structure.
- Flexibility and Ease of Fabrication: Polymers are flexible materials that can be easily processed into thin, defect-free membranes using various fabrication techniques. This allows for the production of large-area membranes at relatively low cost.
Advantages and Disadvantages
- Advantages:
- Lower cost compared to palladium-based membranes.
- Flexibility and ease of fabrication.
- Wide range of polymer materials available, allowing for tailoring of membrane properties.
- Disadvantages:
- Lower permeability compared to palladium-based membranes.
- Limited stability at high temperatures and pressures, potentially leading to membrane degradation.
- Susceptibility to swelling and plasticization by certain gases, affecting membrane performance.
Mixed Matrix Membranes (MMMs)
Mixed matrix membranes combine the advantages of both inorganic and polymeric materials to enhance hydrogen separation performance. These membranes typically consist of a polymer matrix containing dispersed inorganic fillers, such as zeolites, carbon nanotubes, or metal-organic frameworks (MOFs).
Properties and Characteristics
- Enhanced Permeability: The inorganic fillers in MMMs can provide pathways for faster hydrogen transport, leading to increased permeability compared to pure polymer membranes.
- Improved Selectivity: The selective nature of the inorganic fillers can further enhance the selectivity of the membrane for hydrogen separation. This results in a more efficient separation process and a higher purity of the separated hydrogen.
- Enhanced Stability: The inorganic fillers can improve the thermal and chemical stability of the membrane, allowing for operation at higher temperatures and pressures.
Advantages and Disadvantages
- Advantages:
- Synergistic combination of inorganic and polymeric materials for improved permeability, selectivity, and stability.
- Tailorable properties by adjusting the type and concentration of fillers in the polymer matrix.
- Potential for cost-effective production using existing polymer processing techniques.
- Disadvantages:
- Challenges in achieving uniform dispersion and compatibility between the inorganic fillers and the polymer matrix.
- Potential for interfacial defects between the filler and polymer, which can affect membrane performance.
- More complex fabrication processes compared to pure polymer membranes.
Applications of Osmosis Hydrogen Membrane Separation
The applications of osmosis hydrogen membrane separation are vast and diverse, spanning various industries and sectors. This technology’s ability to efficiently separate hydrogen from gas mixtures makes it particularly valuable in hydrogen production, purification, and storage, as well as in fuel cell technology and other energy-related applications.
Hydrogen Production
Osmosis hydrogen membrane separation plays a crucial role in enhancing hydrogen production processes. Its ability to selectively permeate hydrogen while effectively rejecting other gases, such as nitrogen, methane, and carbon dioxide, makes it an efficient method for producing high-purity hydrogen. This technology can be integrated into various hydrogen production methods, including steam methane reforming, electrolysis, and biomass gasification.
- Steam Methane Reforming: In steam methane reforming, hydrogen is produced by reacting methane with steam at high temperatures. The resulting gas mixture contains hydrogen, carbon dioxide, and other impurities. Osmosis hydrogen membrane separation can be used to selectively remove hydrogen from this mixture, resulting in a high-purity hydrogen stream.
- Electrolysis: Electrolysis is a process that uses electricity to split water into hydrogen and oxygen. Osmosis hydrogen membrane separation can be employed to purify the hydrogen produced by electrolysis, removing any residual oxygen or other impurities.
- Biomass Gasification: Biomass gasification is a process that converts biomass into a gas mixture containing hydrogen, carbon monoxide, and other gases. Osmosis hydrogen membrane separation can be used to selectively extract hydrogen from this mixture, producing a high-purity hydrogen stream.
Hydrogen Purification
Osmosis hydrogen membrane separation is a highly effective method for purifying hydrogen gas. Its selective permeability to hydrogen allows it to remove impurities such as nitrogen, methane, carbon dioxide, and other gases from hydrogen streams. This purification process is essential for various applications, including fuel cell technology, where high-purity hydrogen is required for optimal performance.
- Fuel Cell Technology: Fuel cells require high-purity hydrogen to operate efficiently. Osmosis hydrogen membrane separation can be used to purify hydrogen produced from various sources, such as steam methane reforming, electrolysis, and biomass gasification, ensuring that the fuel cell operates at optimal performance.
- Industrial Processes: Hydrogen is used in various industrial processes, such as ammonia production, methanol synthesis, and metal refining. These processes often require high-purity hydrogen, which can be obtained through osmosis hydrogen membrane separation.
Hydrogen Storage
Osmosis hydrogen membrane separation can also be used for hydrogen storage. By selectively permeating hydrogen, the technology can be used to concentrate hydrogen from a dilute gas mixture, effectively increasing the hydrogen density. This is particularly useful for storing hydrogen in a compact and efficient manner.
- On-site Hydrogen Storage: Osmosis hydrogen membrane separation can be used to concentrate hydrogen from a dilute gas mixture, such as the exhaust gas from a fuel cell vehicle, allowing for on-site hydrogen storage.
- Hydrogen Storage for Transportation: Hydrogen can be stored in a more compact and efficient manner by using osmosis hydrogen membrane separation to concentrate hydrogen from a dilute gas mixture, making it suitable for transportation applications.
Fuel Cell Technology
Osmosis hydrogen membrane separation plays a crucial role in fuel cell technology. Its ability to purify hydrogen to high purity levels ensures optimal performance and efficiency of fuel cells. This technology is particularly important for applications where high-purity hydrogen is essential, such as in portable fuel cells, automotive fuel cells, and stationary fuel cells.
- Portable Fuel Cells: Portable fuel cells are used in various applications, such as powering electronic devices, providing backup power, and supplying electricity to remote locations. Osmosis hydrogen membrane separation can be used to purify hydrogen from a variety of sources, ensuring that the fuel cell operates efficiently and reliably.
- Automotive Fuel Cells: Automotive fuel cells are used to power vehicles, offering a clean and efficient alternative to traditional gasoline-powered engines. Osmosis hydrogen membrane separation can be used to purify hydrogen produced from various sources, such as electrolysis and steam methane reforming, ensuring that the fuel cell operates efficiently and reliably.
- Stationary Fuel Cells: Stationary fuel cells are used to provide power for buildings, industries, and other stationary applications. Osmosis hydrogen membrane separation can be used to purify hydrogen from various sources, ensuring that the fuel cell operates efficiently and reliably.
Other Energy-Related Sectors, Osmoses hydrogen membrane separation
Beyond fuel cell technology, osmosis hydrogen membrane separation has numerous applications in other energy-related sectors. Its ability to efficiently separate hydrogen from gas mixtures makes it valuable for various processes, including:
- Syngas Production: Syngas is a mixture of carbon monoxide and hydrogen used as a feedstock for various chemical processes. Osmosis hydrogen membrane separation can be used to selectively remove hydrogen from syngas, producing a high-purity hydrogen stream and enriching the syngas with carbon monoxide.
- Carbon Capture and Storage: Osmosis hydrogen membrane separation can be used to capture and store carbon dioxide from industrial processes, such as power plants and cement production. This technology can be used to selectively remove carbon dioxide from flue gas, allowing for its capture and storage.
- Hydrogen-Based Energy Storage: Osmosis hydrogen membrane separation can be used to store hydrogen in a more compact and efficient manner, making it a promising technology for hydrogen-based energy storage systems.
Future Trends and Developments
The field of osmosis hydrogen membrane separation is rapidly evolving, with ongoing research and development efforts focused on improving the efficiency and applicability of this technology. These advancements aim to overcome current limitations and unlock the full potential of this promising approach for hydrogen production and purification.
Advancements in Membrane Materials
The development of new membrane materials with enhanced properties is a key focus area in osmosis hydrogen membrane separation. Researchers are exploring various materials and modifications to achieve higher permeability, selectivity, and stability.
- Metal-organic frameworks (MOFs): These highly porous materials offer excellent surface area and tunable pore sizes, enabling selective hydrogen permeation. MOFs are being investigated for their potential to create membranes with superior performance compared to traditional polymeric membranes.
- Covalent organic frameworks (COFs): COFs are another class of porous materials known for their high stability and tunability. They exhibit promising properties for hydrogen separation due to their well-defined pore structures and robust frameworks.
- Graphene-based membranes: Graphene, a single-atom-thick sheet of carbon atoms, possesses exceptional mechanical strength, electrical conductivity, and impermeability to most gases. Researchers are exploring the use of graphene and its derivatives for creating highly selective hydrogen membranes.
Membrane Design and Fabrication
Alongside advancements in materials, innovative membrane design and fabrication techniques are crucial for improving the performance and scalability of osmosis hydrogen membrane separation.
- Thin-film composite membranes: These membranes combine a thin selective layer with a porous support layer, enhancing both permeability and mechanical strength. Advanced fabrication methods, such as interfacial polymerization and layer-by-layer assembly, are being employed to create these composite membranes.
- Microfluidic fabrication: Microfluidic techniques enable precise control over membrane structure and morphology at the microscale. This allows for the creation of membranes with tailored pore sizes and geometries, enhancing their selectivity and performance.
- 3D printing: 3D printing offers the potential to fabricate complex membrane structures with controlled porosity and surface properties. This technology allows for the creation of membranes with customized designs tailored to specific applications.
Integration with Emerging Technologies
The integration of osmosis hydrogen membrane separation with other emerging technologies, such as renewable energy sources and carbon capture technologies, can create synergistic benefits and enhance the overall efficiency of hydrogen production and utilization.
- Renewable energy integration: Combining osmosis hydrogen membrane separation with renewable energy sources, such as solar or wind power, can enable the production of clean and sustainable hydrogen. This integration can create a closed-loop system that minimizes environmental impact.
- Carbon capture and storage (CCS): Integrating osmosis hydrogen membrane separation with CCS technologies can facilitate the capture and sequestration of CO2 emissions generated during hydrogen production. This integration can significantly reduce the carbon footprint of hydrogen production.
- Hydrogen storage and transportation: Advancements in hydrogen storage and transportation technologies, such as high-pressure tanks and liquid hydrogen storage, can enhance the efficiency and practicality of osmosis hydrogen membrane separation for various applications.
Osmosis hydrogen membrane separation, a technology brimming with potential, offers a promising path towards a cleaner and more sustainable energy future. While challenges remain, ongoing research and development efforts are pushing the boundaries of this technology, paving the way for widespread adoption across diverse industries. As we strive for a greener tomorrow, osmosis hydrogen membrane separation stands as a beacon of innovation, a testament to the transformative power of science and engineering.
Osmosis hydrogen membrane separation is a fascinating process, but it’s not the only innovative technology changing the world. For instance, earlybird raises 4 5m scale fintech gifting platform for kids is revolutionizing how we approach financial literacy and gift-giving. While hydrogen membrane separation focuses on clean energy, earlybird aims to empower the next generation with financial tools, proving that innovation comes in many forms, each contributing to a brighter future.
 Standi Techno News
Standi Techno News